by Chelo S. Pascua
National Institute for Materials Science, Tsukuba, Ibaraki, Japan
 The mobility of aqueous inorganic arsenic in the geothermal environment is influenced by the (1) aqueous arsenic speciation, (2) affinity of arsenic aqueous species to minerals, and (3) stability of mineral host. These factors also dictate arsenic contamination susceptibility in areas with naturally high arsenic concentrations such as in geothermal fields. There are two common inorganic forms of arsenic – the trivalent arsenite species deemed more toxic than the pentavalent arsenate species.
The mobility of aqueous inorganic arsenic in the geothermal environment is influenced by the (1) aqueous arsenic speciation, (2) affinity of arsenic aqueous species to minerals, and (3) stability of mineral host. These factors also dictate arsenic contamination susceptibility in areas with naturally high arsenic concentrations such as in geothermal fields. There are two common inorganic forms of arsenic – the trivalent arsenite species deemed more toxic than the pentavalent arsenate species.
Arsenic aqueous speciation is greatly influenced by redox, pH and to a certain extent temperature. At reduced conditions arsenite (As3+) species dominate while arsenate (As5+) species are prevalent in oxidized conditions. At pH>9, arsenite species auto-oxidizes to arsenate species. In geothermal systems where dissolved sulfide abound, arsenite species such as H3AsO3(aq) and H2AsO3– are present at higher temperatures (T>150°C) while arsenite sulfides form at lower temperatures. Sulfide minerals (e.g. pyrite, arsenopyrite, orpiment and realgar) are common in the geothermal environment.
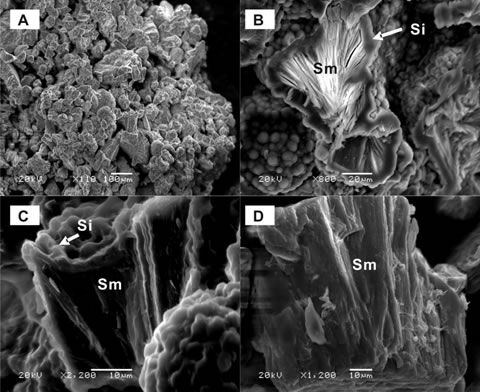 |
| Figure 1. Scanning Electron Microscopy (SEM) images of the arsenic-bearing smectite (Sm). (A) Sm particles cemented by amorphous silica (Si); (B and C) at higher magnifications, Si coats the Sm; (D) Sm occurs as uncoated laths. |
In a geothermal field in northwestern Japan, a phyllosilicate mineral called stevensite (Mg-rich smectite clay mineral) was found to host a large amount of arsenic (Figure 1; Pascua et al., 2005). Based on selective chemical extractions, X-ray diffraction and SEM-EDS analyses, infrared spectroscopy, and X-ray spectroscopy (i.e. X-ray Photoelectron Spectroscopy and synchrotron-based X-ray Absorption Spectroscopy); arsenic is dissolved in the smectite structure. This is the first report of an arsenic-rich smectite.
 |
| Figure 2. A SEM image of the retrieved silica via lime addition in a Seed Addition and Circulation System (SACS). Arrow points to a lath shaped smectite particle. |
Fe-hydroxides and to a certain extent the clay mineral kaolinite were found to be the arsenic sinks in a Philippine geothermal field (Pascua et al., 2006). Fe-hydroxides are secondary precipitates formed from the oxidative dissolution of Fe-sulfide minerals and occur as small pockets in natural geothermal sinter. Despite their limited occurrence in geothermal systems, they are the main hosts for aqueous arsenate. Most of the documented arsenic contamination is related to the reductive dissolution of Fe-(oxy)hydroxides.
Lime (Ca(OH)2) addition is the most economical way of inducing dissolved silica precipitation to retrieve silica from spent geothermal brine (Figure 2). However, excess calcium causes arsenate precipitation in the retrieved materials. It was necessary to initially remove dissolved arsenic prior to precipitation of silicates via lime addition. Amongst several alternative sorbents investigated, synthetic schwertmannite [Fe8O8(OH)5.25(SO4)1.375] was found the most effective in removing arsenic without affecting dissolved silica (Pascua et al., 2007). Arsenate has a higher affinity towards Fe-hydroxides while arsenite does not exhibit any affinity to most available sorbent. The effectivity of synthetic schwertmannite lies in its ability to facilitate dissolved arsenite (As3+) oxidation to arsenate which is simultaneously taken up by schwertmannite.
References:
- Pascua, C.S., Minato, M., Yokoyama, S., Sato, T. 2007. Uptake of dissolved arsenic during the retrieval of silica from spent geothermal brine. Geothermics, 36, 230-242.
- Pascua, C.S., Sato, T., Golla, G. 2006. Mineralogical and geochemical constraints on arsenic mobility in a Philippine geothermal field. Acta Geologica Sinica, 80, 230-235.
- Pascua, C., Charnock, J., Polaya, D.A., Sato, T., Yokoyama, S., Minato, M. 2005. Arsenic-bearing smectite from the geothermal environment. Mineralogical Magazine, 69, 897-906.
__________
Chelo S. Pascua obtained his BSc. and MSc. degrees in Geology from the National Institute of Geological Sciences, University of the Philippines, Diliman, Quezon City in 1997 and 2001, respectively. In 2006, he obtained his Ph.D. in Environmental Mineralogy and Geochemistry from Kanazawa University, Japan. He is currently a postdoctoral researcher in the National Institute for Materials Science, Tsukuba, Ibaraki, Japan.
Previous research involvement include topics in geology, hydrogelogy, engineering geology, remote sensing and GIS, acid mine drainage, clay mineral dissolution, and arsenic contamination and remediation. His current work is focused on synthetic clay minerals for applications in clay-polymer nanocomposites, band tunable (ultra)thin films, clay-based photocatalysts, and liquid crystal mineral moieties.