Manolito E. Bambase, Jr.
Life Sciences and Bioengineering, Graduate School of Life and Environmental Science, University of Tsukuba, Tsukuba City, Ibaraki, Japan
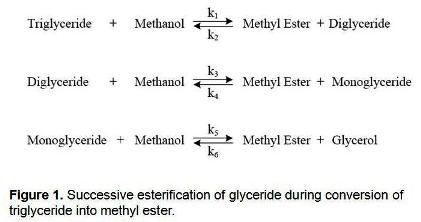
 In recent years, increased attention has been given to the use of biodiesel as a potential substitute for petroleum diesel mainly due to its renewability and being a cleaner burning fuel. It can be produced from any triglyceride-containing material such as animal fat or vegetable oil. It is commonly obtained via a transesterifcation reaction in which the triglyceride is reacted with methanol in the presence of a hydroxide catalyst producing methyl esters (chemical name for biodiesel) and a glycerol by-product. The reaction proceeds as three successive and reversible reactions in which di- and monoglycerides are formed as intermediate products (figure 1).
In recent years, increased attention has been given to the use of biodiesel as a potential substitute for petroleum diesel mainly due to its renewability and being a cleaner burning fuel. It can be produced from any triglyceride-containing material such as animal fat or vegetable oil. It is commonly obtained via a transesterifcation reaction in which the triglyceride is reacted with methanol in the presence of a hydroxide catalyst producing methyl esters (chemical name for biodiesel) and a glycerol by-product. The reaction proceeds as three successive and reversible reactions in which di- and monoglycerides are formed as intermediate products (figure 1).
 |
Understanding the kinetics of the biodiesel reaction can provide important insights into its behaviour when subjected to different conditions which can then serve as a basis for designing a more efficient and cost-effect production system. Hence, kinetic studies were conducted to determine the influence of temperature, mixing intensity, catalyst loading, and reactants molar ratio on product yield using crude sunflower oil as raw material and sodium hydroxide as catalyst.
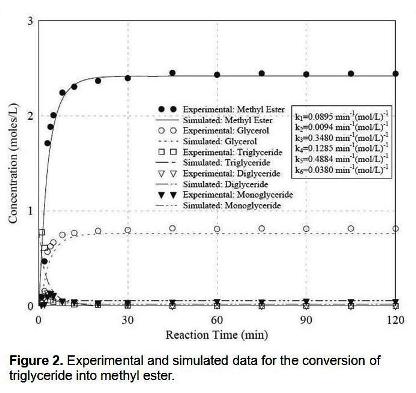 |
Experimental results have shown that during the initial stage of the reaction, the most critical parameter is mixing intensity to minimize mass-transfer limitations. As the reaction progresses, temperature becomes the most influential fator affecting the rate and extent of the transesterification reaction. The hydroxide catalyst must also be present in sufficient quantity to avoid premature termination of the reaction as the catalyst is consumed via the saponification side reaction. Increasing the methanol:oil mole ratio resulted in a more rapid formation of the methyl ester although no practical difference was measured with regard to the final yield. A system of second-order different equations was simulateneously fitted to the experimental data to estimate the kinetic rate constants using standard fitting procedures to model the progress of the reaction in terms of product formation. A typical plot for the experimental and simulated data is shown in figure 2. From the kinetic rate constants, values of the activation for the individual steps of the transesterification reation were determined and found to agree satisfactorily with previously reported values.
Reference:
M. Bambase, Jr, N. Nakamura, J. Tanaka, and M. Matsumura, “Kinetics of hydroxide-catalyzed methanolysis of crude sunflower oil for the production of fuel-grade methyl esters,” J. Chem. Technol. Biotechnol. 82(2007) 273-280.
__________
Manolito E. Bambase Jr. is currently a PhD student at the Graduate School of Life and Environmental Science, University of Tsukuba, Japan. His research is on the design and optimization of production process for the conversion of vegetable oil into biodiesel via hydroxide-catalyzed transesterification. He obtained his Master of Biosystem Science in March 2005 from the Graduate School of Biosystem Studies, University of Tsukuba and his Bachelor of Science in Chemical Engineering in March 1998 from the College of Engineering and Agro-Industrial Technology, University of the Philippines Los Baños.